 |
OSOM® Ultra Plus Flu A&B Test
SEKISUI DIAGNOSTICS
The OSOM® Ultra Plus Flu A & B Test by Sekisui is an in vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab and nasopharyngeal swab specimens obtained from patients with signs and symptoms of respiratory infection.
|
|
OSOM® Ultra Plus Flu A&B Test Stronger Clinical Performance Takes Lateral Flow Testing To The Next Level Providing superior rapid results at the point-of-care. Fast, easy, cost effective so you can test and treat in one visit.
The OSOM®Ultra Plus Flu A & B Test is an in vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab and nasopharyngeal swab specimens obtained from patients with signs and symptoms of respiratory infection.
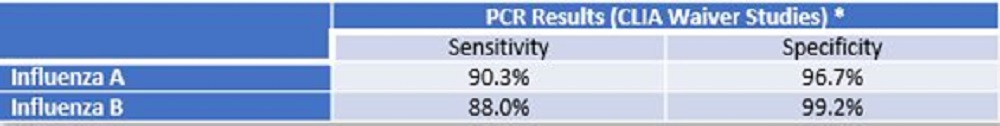
*Refer to the Package Insert for additional performance claims.
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
|
 |
BD Veritor System
BD Diagnostics
BD - Diagnostic Systems: BD Veritor System: CLIA-waived For Rapid Detection of Flu A+B.
|

BD Veritor ™ System CLIA-waived for Rapid Detection of Flu A+B meets the New FDA Requirements Our BD Veritor ™ line of immunoassay products, the BD Veritor ™ Plus System and the BD Veritor ™ System give healthcare providers and laboratorians in near patient settings the objective, lab-quality tests results at the point of care within minutes. This assay system eliminates the subjectivity of visual interpretations of tests results and replaces it with an objective digital result. BD Veritor ™ System – Proven Performance vs. Polymerase Chain Reaction (PCR)
Summary of the Performance Data for the BD Veritor System for Rapid Detection of Flu A+B tests compared to PCR for all swabs – all sites 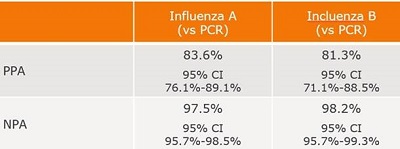 Convenient Workflow 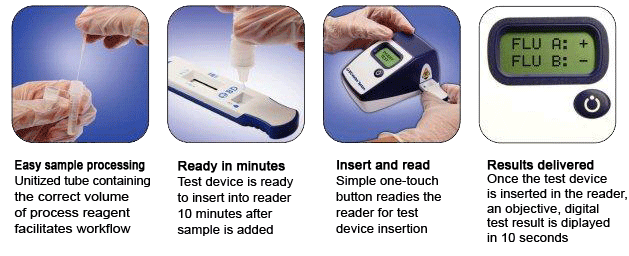 Changing The Way You View Rapid Testing 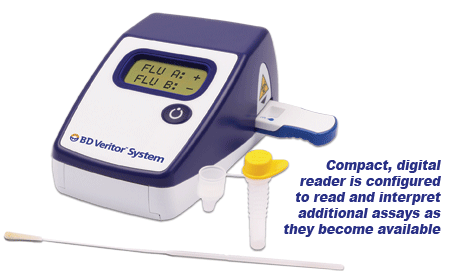
BD Diagnostics
7 Loveton Circle | | Sparks | MD |
BD - Diagnostic Systems: BD Veritor System: CLIA-waived For Rapid Detection of Flu A+B

|
|
 |
cobas® Influenza A/B assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes
|
|
cobas® Influenza A/B assay The cobas® Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes. Available for use in non-traditional testing sites, including ERs, physician offices, pharmacy clinics and other urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
|
 |
cobas® Influenza A/B & RSV assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes
|
|
cobas® Influenza A/B & RSV assay The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes. It's available for use in hospitals, physician offices and urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
|
 |
BioFire FilmArray® Torch
BioFire Diagnostics
The BioFire® FilmArray® Torch is a fully integrated, random, and continuous access system designed to meet your laboratory’s syndromic infectious disease testing needs
|
|
The BioFire® FilmArray® Torch is a fully integrated, random, and continuous access system designed to meet your laboratory's syndromic infectious disease testing needs. The BioFire Torch offers a radically reduced benchtop footprint, saving precious space in the lab, and its scalability meets high throughput demands. BioFire® FilmArray® Link Software automatically uploads patient results. Fully compatible with all CLIA Moderate BioFire® FilmArray® Panels, the BioFire Torch helps you maximize efficiency and productivity.
BioFire Diagnostics
515 Colorow Dr | | Salt Lake City | UT |
BioFire simplifies our customers’ lives by innovating easy-to-use clinical molecular diagnostic solutions that provide fast and accurate results.

|
|